Alpha
Radiation - Practically nuclei of helium atoms consisting
of 4 particles, i.e. 2 neutrons and 2 protons. Of
greater mass than beta and gamma particles, they react more
promptly in traversing the atmosphere or denser materials hence a
few centimeters of air will suffice to dampen them. They can hardly
penetrate the external cellular tissue of the epidermis but if ingested
the menace they pose is much greater, tenfold the effects of beta
particles.
RADIOACTIVITY AND THE ORGANISM [1]
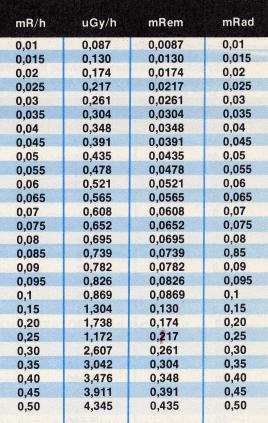
The Roentgen [2] , so called in honor of W. K. Roentgen, the discoverer of the X-rays, represents the specific energy amount used by X-rays, gamma and beta radiation when they ionize one gram of air. Following the ionization of 1 Roentgen an amount of about 94 ergs [3] of energy will accumulate in one gram of soft tissue in the body. Hence a radiation meter calibrated in roentgen will indicate the radioactivity in the air close to the radiation meter. The amount of the energy absorbed by the live tissue will be about 14 percent greater then the absorbency/ionization of the air.
This
means that, if the radiation meter (or RADIAC) shows
an amount of 10 roentgen in the air, our probable absorbency will
be 11.4 roentgen. With 100 roentgen in the air, the absorbency
will be 114 roentgen, and with 400 roentgen in the air the organism's
absorbency will be 456 roentgen which, in the last case, will
easily result as a lethal radiation dose. |
[2]
A unit of radiation exposure; the dose of ionizing radiation that will
produce 1 electrostatic unit of electricity in 1 cc of dry air.
[3]
A cgs unit of work or energy; the work done by a force
of one dyne acting over a distance of one centimeter.